Using CFPS to optimize the synthesis of Immunoglobulin A
I've been researching breastmilk for a few months now, for the purpose of figuring out a way we can produce this extremely complex fluid outside of the human body, or at least synthesize some of it's components.
Breastmilk contains around 2,500 different components. This is mostly water (87%), carbs, primarily lactose (7%), fats (4%), and proteins (1%). There are many different types of proteins, but antibodies are arguable the most important for the immune system.
Secretory Immunoglobulin A (sIgA) makes up about 90% of all antibodies in breastmilk. It acts as a barrier between the stomach lining. and bacteria that enters the body.
I started out by proposing the synthesis (production) of sIgA by using agrobacterium-mediated gene transfer to grow them in transgenic tobacco plants.
It would be difficult to predict the production of antibodies in these plants without testing the process. However, testing the process at full-scale is also extremeley expensive.
Specifically, purifying the proteins and maintaining the plants are the largest cost factors. Therefore, cell-free protein synthesis (CFPS) is more applicable during the testing process. There are only a handful of cell components that are responsible for over 80% of producing the antibody. CFPS is a system that produces proteins using this principle. Instead of using the entire cell, the neccessary components are extracted during cell lysis and used to synthesize the desired protein.
Regular in vitro protein production
Generally, in order to synthesize a protein, DNA is incerted into a cell using gene transfer performed by a plasmid (circular peace of DNA carrier in bacteria).
The cell goes through transciption + translation to produce the protein, after which it is lysed (ripped apart) and the protein is purified.
Unfortunately, there are several disadvantages to this process, including slow protein growth, expensive purification, toxic proteins, poor yields and high monitoring.
Cel-Free Protein Synthesis (SFPS)
(This is what everything's been leading up to)
Cell lysates provide the correct composition and proportion of enzymes and building blocks required for translation. (Usually, an energy source and amino acids must also be added to sustain synthesis.) Cell membranes are removed to leave only the cytosolic and organelle components of the cell (hence the term, "cell-free extracts"). The first types of lysates developed for cenisms. More recently, systems based on extracts from insect cells, mammalian cells and human cells have been developed and made commercially available.
Cell-free protein synthesis
Because there are really only a few components of the cell that operate to synthesize proteins, the entire cell isn't required for this process.
There are two components required for in vitro protein expression:
- The genetic material (mRNA or DNA) encoding the target protein (in this case its an antibody)
-
A solution that contains the molecules that will do the transcription and translation (from genetic material to protein). These include:
-
RNA polymerases for mRNA transcription (the very first step)
- ribosomes for polypeptide translation
- tRNA and amino acids
- enzymatic cofactors and an energy source for the process
- in some cases other cellular components essential for proper protein folding
How these components are gathered:
- Live cells are grown in liquid culture (just normal cell growth)
- The cells are harvested out of the culture
- The cells are busted open— usually done by vibrating the cells until they explode. Then gather the cellular components that are required for protein synthesis (as explained above).
Once the DNA is introduced to into the lysed cell, the process begins. About 2 hours later, the proteins can be extracted and purified.
There are several different types of cells that can be used for cell-free protein synthesis, each have advantages + disadvantages.
Secretory Immunoglobulin A is a higher order protein complex that is formed by several different parts that come together. One of the processes involved in this is called glycosylation, a process by which a sugar is attached to a part of the protein to help it properly fold.
There are only certain cells that are able to perform glycosylation — most of which are Eukorotic cells.
There is also the endoplasmic reticulum (ER), which is only present in Eukaryotic (animal) cells. The ER is responsible for part of the protein folding process — it creates an oxidative (lacking oxygen) environment, which helps form + stabilize disulphide bonds in the antibody.
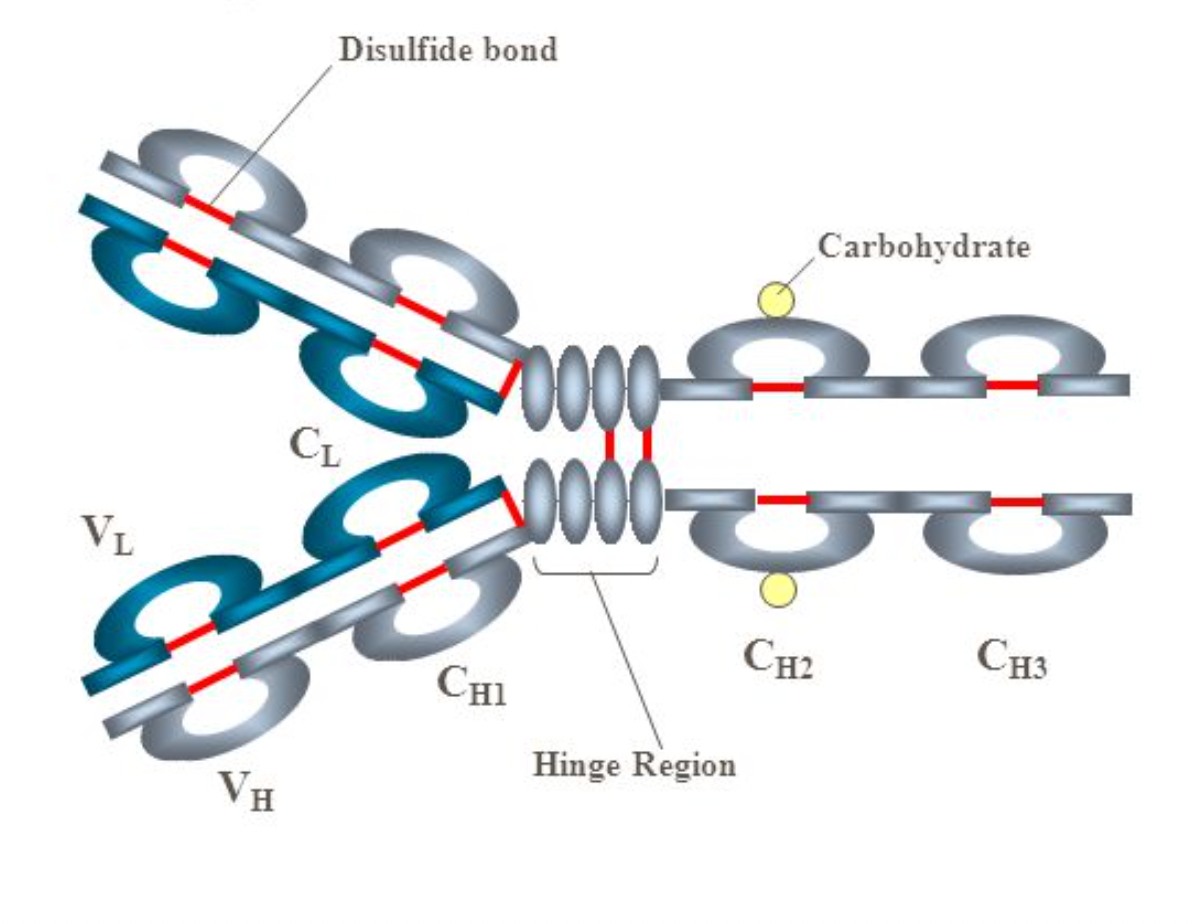
The red lines show disulfide bonds.
Another protein, called BiP helps make sure that the heavy chain (at the bottom of the antibody) doesn't unfold. Prolyl isomerases have also been proven to be involved in catalyzing the protein folding step.
Once the protein is folded, the purification process begins. Because the number of cellular components in a CFPS system is significantly less compared to a regular system, the protein purificaiton process is cheaper and faster.
Open-cell-Free Synthesis
Open-cell-free synthesis is a system which allows to addition of certain components that are foreign to the cell to improve protein folding.
This process has been done for Immunoglobulin G, where each of the following chaperones (components that can aid in protein folding) were tested to determine which improves expression the best.
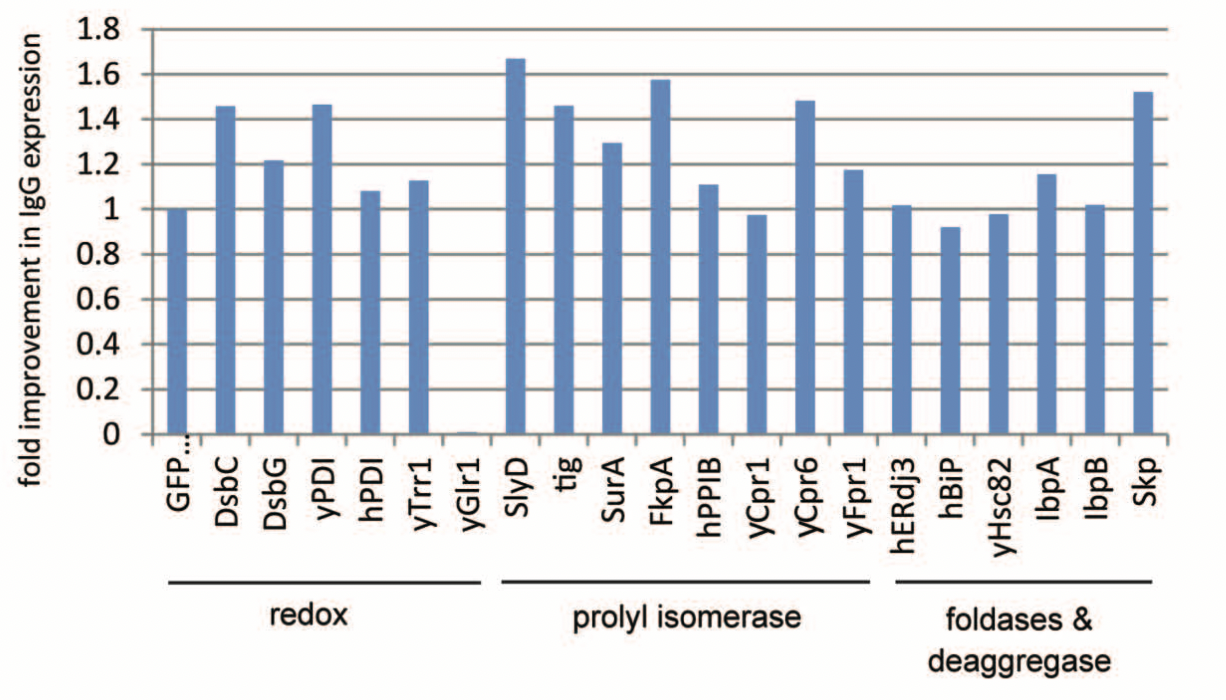 Using a trial and error approach with this cell-free system, we are able to determine the components
that lead to a higher yield and better expression of the antibody. Adding different chaperones to the
cells can improve qualtiy protein yield by 40%. And therefore, this system allows us to optimize the
biofactories at a much smaller, less expensive, more controlled and faster scale.
Using a trial and error approach with this cell-free system, we are able to determine the components
that lead to a higher yield and better expression of the antibody. Adding different chaperones to the
cells can improve qualtiy protein yield by 40%. And therefore, this system allows us to optimize the
biofactories at a much smaller, less expensive, more controlled and faster scale.
The gene encoding for the over-expression of the desirable components is then inserted into the genome of the cells for product development and eventually manufacturing.
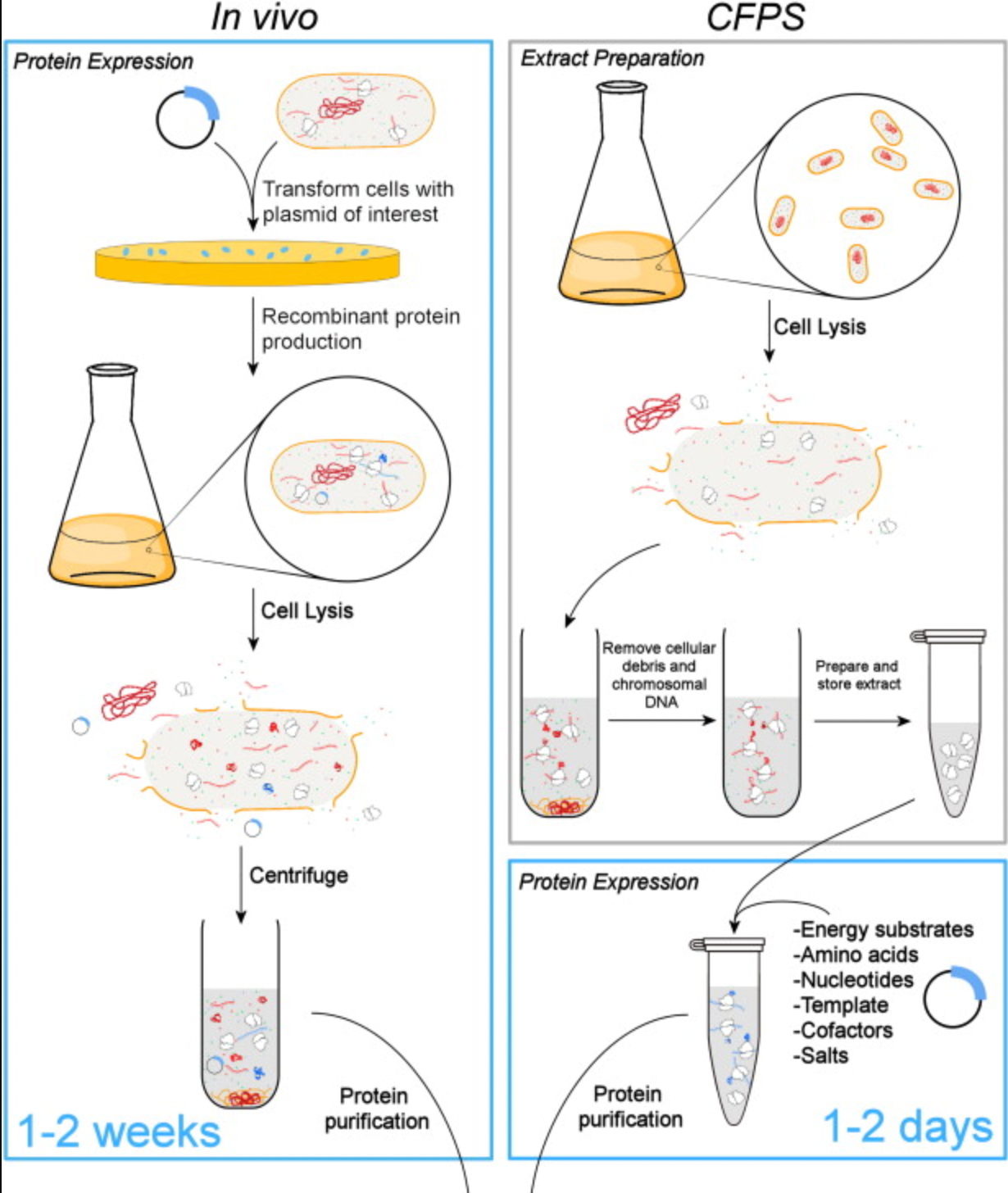
The following is diagram comparison of regular protein synthesis, vs cell-free protein synthesis
And on that note, that's the end of my thought process around cell-free protein synthesis. If you have any questions or thoughts, feel free to reach out, I'm more than happy to have a conversation!