Bacterial engineering to solve cancer.
Cancer was around for 243 million years before the first humanoids came to life, and since then, has been steadily responsible for 10–15% of human death. In fact, the first malignant tumor was found in a 240 million-year-old turtle bone, which suggests that the disease has been around for at least, that many years.

The first person to give a name to Cancer in 400 BC was Hippocrates. Using the words “carcinos” and “carcinoma”, which meant “crab” in greek, he defined non-ulcer forming and ulcer-forming tumors. The greek word was then translated into Latin and hence, we know the disease today as “cancer”. Now the reason I’m telling you this, isn’t because this is an article on cancer and I’m hence, explaining the history. People have understood that cancer is a “thing” since 400 BC, and still, with so many smart people in our world, we have not cured it.
That doesn’t make sense to me.
Cancer research has gone through several steps over the past 300 years, and here’s an overview.
- First discovered through epidemiology There were several instances throughout the 17th and 18th century where high cancer rates were notices in specific groups of people. For example, lung cancer was very common among miners in Europe, and “throat” cancer was concluded to be associated with cigar smoking.
Conclusions made:
- Cancer was impacted by external factors, most of which were preventable.
- Since many diseases during this time were caused by bacteria, it was established that cancer was also infectious
Questions needed to be answered:
- Why were these different external factors causing cancer?
2. Advancements in cell biology
Scientists began studying cancer in labs using microscope and analyzing their behaviors in an organism.
Conclusions made:
- Cancer cells were established to be different that regular cells, and seemed to replicate at an insane speed.
- Scientists understood that normal cells underwent genetic mutations to become a cancerous cell, and therefore, any cell in the body could become cancerous. Cancer, then, is a disease in which a single normal body cell undergoes a genetic transformation into a cancer cel.
Questions need to be answered:
- Why do cancer cells develop in certain type of cells?
- How are cancer cells different from regular cells and what mutations they undergo?
- Why do cancer cells proliferate at such intense speed? What genes are involved in the process?
- How do their functions of these cells change as they mutate?
3. Today’s Medicine
“Cancer” is now a term used for over 100 different diseases, each of them having distinct symptoms, features and treatments. The variation from one cancer to another is the reason why we can’t find a one-fits all cure and why it took so long to understand. However, in the most basic way, cancer occurs when a cell “glitches” and begins to divide uncontrollably, while also, not performing any nessacary functions for our body.
Cancer will develop over a long period of time, undergoing succession in the form of genetic changes. Triggered by the disfunction of the only a couple of genes, it also only starts with the mutation of the genetic code of a single cell.
There’s a common misconception that everyone has cancerous cells in their bodies. While this isn’t completely false, it’s also not entirely true. Any cell has the possibility of becoming cancerous if it has the ability to:
- divide → a process called mitosis, which is essential for most cells in our body
- make mistakes → specifically pertaining to the genetic sequence of the cell
The levels of cellular organization show that:
Organism → Organ System → Organ → Tissue → Cell
Which means that the cell is the building block of everything contained in our bodies. If we break it down even further:

DNA (Deoxyribonucleic Acid) is the structure that has encoded instructions, which responsible for our appearance, how we survive, how we react to the external environment and other characteristics.
And genes are long snippets of DNA. There are two broad classes of genes that control the growth in a cell: proto-oncogenes and tumor suppressor genes. Together, these genes coordinate the cell cycle, and therefore, will also contribute to the avoidance of uncontrollable growth and tumor formation /development.
Proto-Oncogenes will stimulate cell division, inhibit cell differentiation and halt cell death.
Example: the ras gene is a proto-oncogene that is involved in molecular pathways that receive growth-stimulating signals from other cells.
Oncogenes are just mutated Proto-oncogenes.
Example: the mutant rasD oncogene derived from ras proto-oncogene, who provides an excessive or uncontrolled growth-promoting signal.
Tumor supressor genes encode proteins that inhibit cell proliferation. Mutations will deactivate these proteins, which deletes the “stop signal” for cell division.
A back-up plan
In addition to those main gene classes, there are a couple other cell systems that help avoid uncontralable cell division.
DNA repair system
Our gene’s are constantly under attack, caused by things like carcinogens (cancer promoters) from the environment and chemicals produced by the cell. As a result, often, errors will occur uding replications. To prevent further damage when this happens, these errors are corrected by the DNA repair system of the cell.
For example, in the diagram below, a cytosine base is converted into uracil, by a process called deamination. A group of enzymes called glycosylases will follow the steps below to fix this error.
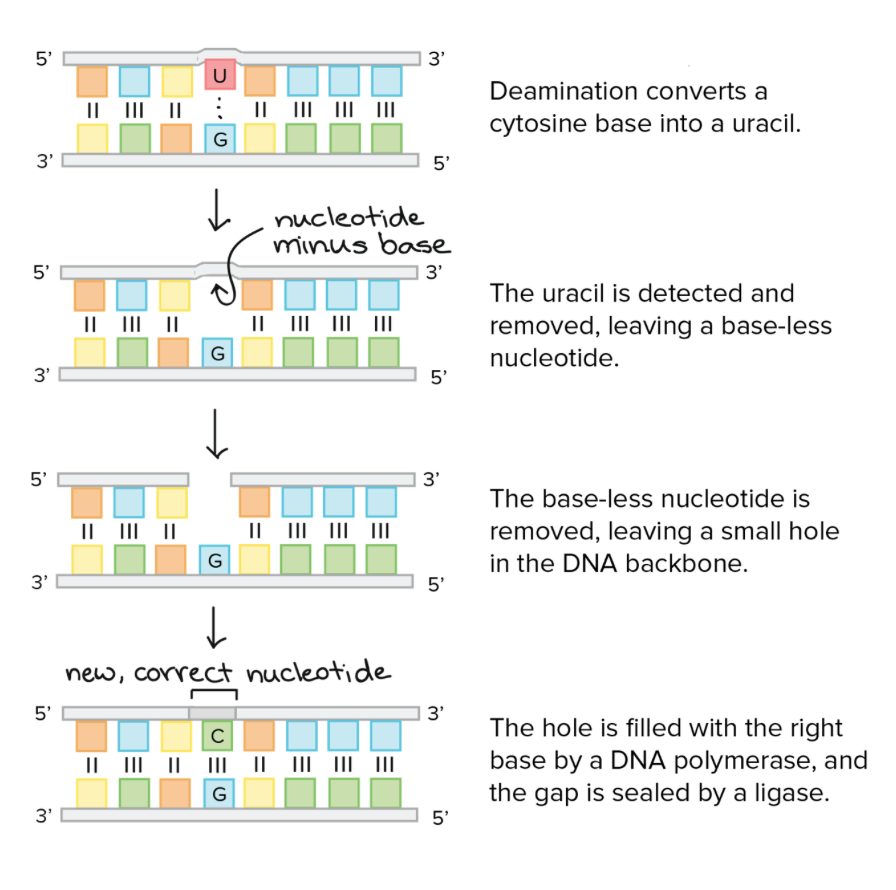
Apoptosis — Cell suicide
The TP53 gene, also known as the “caretaker” gene encodes the proten p53. In its natural form, this protein will induce apoptosis in mutated or abnormal cells. If a tumor manages to inactivate the p53 protein, then the cell will escape this death. This is harmful for two reasons: it contributes to tumor growth and makes cancer cells resistant to treatment.
We used to believe that ratidation + chemotheraphy directly killed cancer cells. However, therapy only damages cell DNA, and because of the way p53 works, the cell will then kill itself. This basically shows that cells that have turned off p53 aren’t too responsive to the treatment.
Telomeres
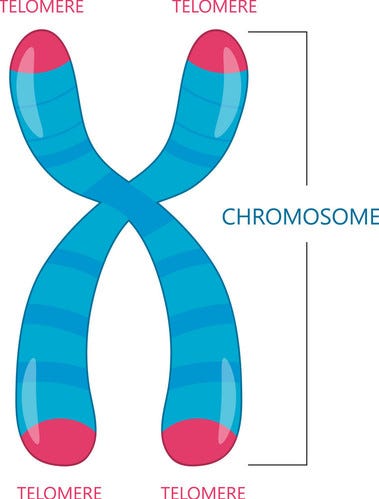
These structure induce a limit on the number of times a cell can divide, since after too many replications, a cell can’t properly replicate its DNA anymore.
Telomeres are located at the ends of chromosomes, and will shorten every time it is replicated. Once the telomeres reach a specific threshold length, the cell will stop dividing.
But if all of this fails, which in most cases, it does, cancer will grow to form tumors, and potentially even spread to the entire boy (metastatic cancer).
Recent advances in targeted therapyand immunotherapy have definitely raised hope for a cure, but still, alot of the time, they either do not work (similar to the apoptosis or cell suicide example), or the cancer will come back.
Programming Bacteria
Scientists have been thinking about using bacteria as an anticancer agent for over 100 years, when a couple of physicians noticed regression of tumors in cancer patients after accidental infections of erysipelas. Erysipelas is a bacterial infection caused by Streptococcus Pyogenes, a bacterium.
A few years after this, another physician injected his patients with heat-killed bacteria (inactivated bacterial cells with preserved surface structures) in effort to stimulate the body’s “resisting powers.” When our body detects bacteria in a specific area, our weaponry system is turned on and the immune system begins to attack the infected tumor.
Although Coley, the physician who made this discovery was seeing remarkable results in certain patients, his method was rejected because it was inexplainable. Coincidentally, the rise of the radiation therapy was hapenning, and that method seemed to be more reliable. So the idea was scratched, but now, we can actually begin to explain why this works and prove that it is a viable treatment.
Using live, natural bacteria — why it theoretically should work
When introduced into the human body, live bacteria will disperse to both cancerous and healthy tissues. However, because tumors are very biochemically unique, bacteria has the capability to grow and thrive inside the tumor’s hypoxic and acidic environment, that also decreased cell surveillance. In comparison, bacteria in healthy tissues will clear much faster.
Theres a couple different ways that bacteria will actually act to destroy the tumor, and every bacteria acts different in every tumor.As the bacteria multiplies, it basically activates immune infiltration, which in simple terms is a proinflammatory process (caused by T cells and cytokine molecules that promote inflimation), which results in immune activation and leads to anti-cancer responses.
For example, Listeria is a bacteria that infects antigen-presenting cells (APCs), which are responsible for cellular immune response, as well as myeloid-derived suppressor cells (MDSCs), which have strong abilities to prevent T-cell responses and expand during cancer. As it overpowers the MDSC cells, its basically able to avoid immune cell clearance, yet will stimulate an immune response.
In theory, this should work, and in some cases it does, however, an incosistant treatment != good treatment and therefore, cannot be used in practice. However, in the cases that it does work, the effectiveness of bacteria is 10x better than that of other, current treatments. Considering that, what we can do is genetically engineer anaerobic bacteria to be enhanced + more reliable.
Here’s a couple factors that we could change to do so:
Cytotoxic agents — responsible for killing cancer
If natural, non-engineered bacteria is not incredibly effective at killing cancerous cell, the most logical thing is to increase the effectiveness of the “thing” that does the killing. So that’s exactly what we do.
A plasmid is a small circular DNA that can replicate independently from chromosomal DNA within a cell. A vector is a more general term that we use to describe a vehicle that researchers use in the laboratory to introduce a DNA of interest into a host cell. A plasmid is a specific example of a vector.
We engineer bacterial vectors to express cytotoxic agents (substances that kill cancer), as well as that can exert greater bystander effect on the surrounding uninfected tumor cells. Then, these vectors can either:
a. be engineered to perfectly target tumors
b. have inducible promoters to better control the gene expression of the bacteria, so that it doesn’t bring harm to normal tissues.
Promoter — the DNA that is needed to either turn a gene on or off. Promoter regions point to where the process of transcription needs to be initiated.
Bacterial toxins are the most commonly used cytotoxic agents in this case, because these genes are native to bacterial physiology.
For example, Cytolysin A (ClyA) is a bacterial toxin that acts by forming pores in cell membranes, which then results in a hypotonic cell and induces apoptosis.
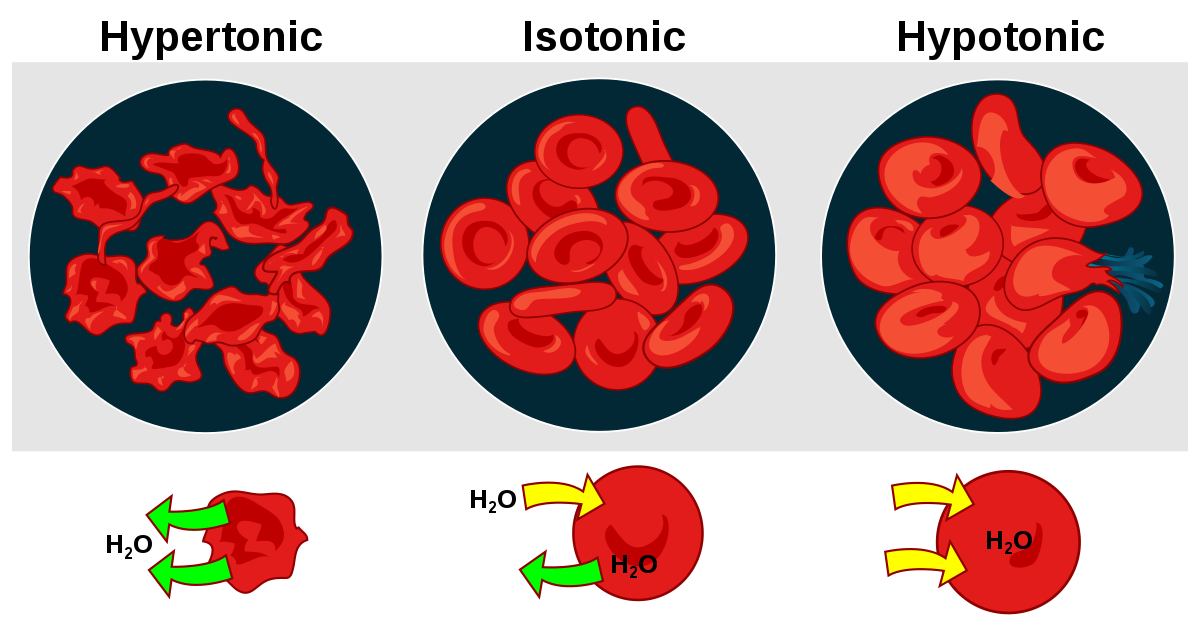
ClyA is a native bacterial protein that is easily transported to the bacterial surface and secreted without much modification.
There are several other examples of cytotoxic agents, including: FAS ligand (FASL), TNF-related apoptosis-inducing ligand (TRAIL) and TNFα. All three of these proteins selectively induce apoptosis and are cytotixic to only cancer cells. However, when systemically administered as protein drugs, all three members of this family have two deficiencies that are overcome by bacterial delivery: hepatotoxicity (liver damage) and a short circulatory half-life (ware off quickly). Producing these proteins in situ (directly in the bacteria) would maintain a higher continual concentration in tumors compared to delivery to the circulatory system , and would reduce the systemic toxicity associated with their administration as small molecules.
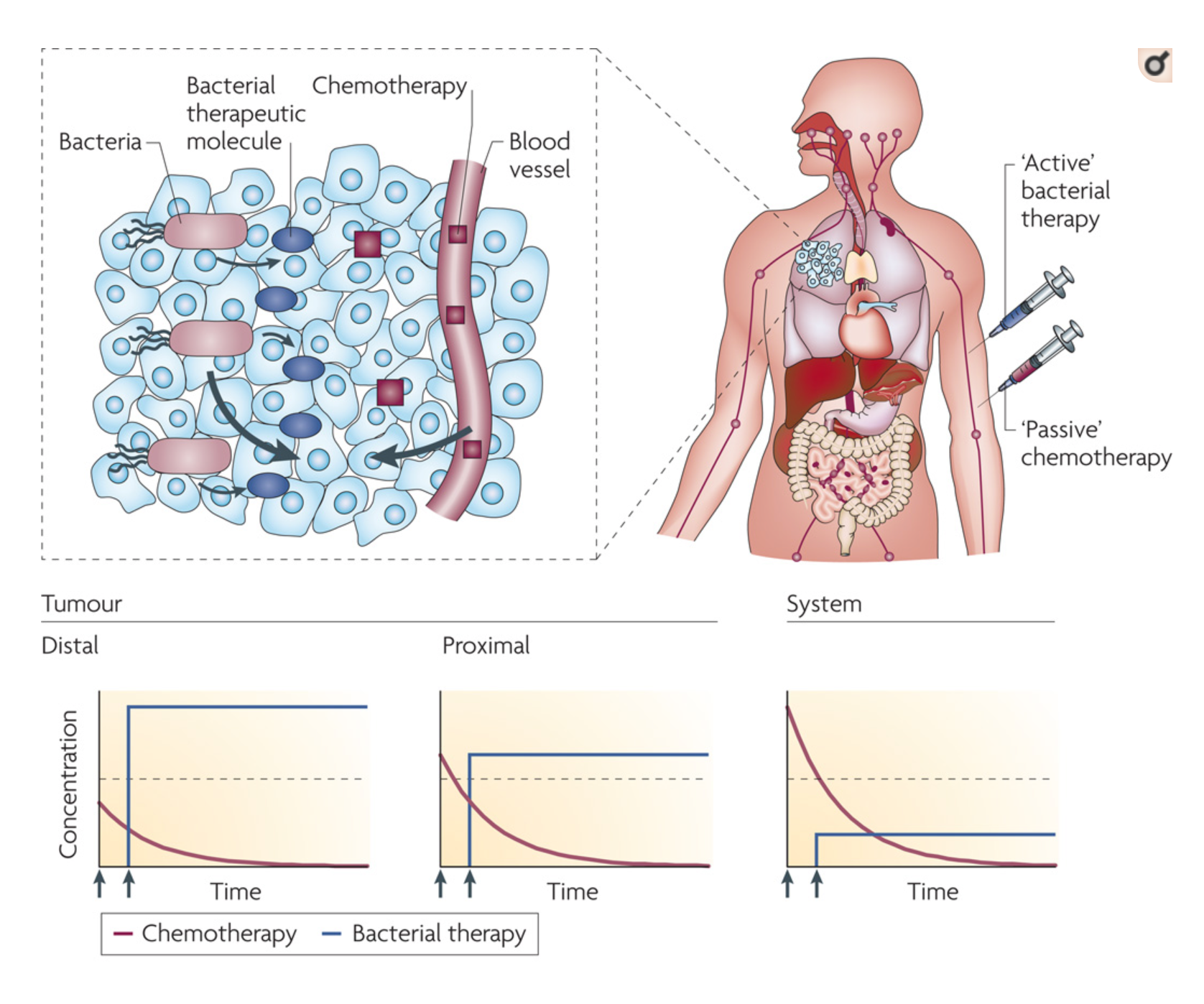
When injected systemically, bacteria (blue syringe, red bacterial organisms), specifically accumulate in tumors and migrate to distal regions that are hypoxic (low oxygen) and hypoglycemic (low blood sugar) Once triggered, bacteria begin to produce therapeutic molecules (blue ovals) that diffuse into the tissue around (clear/light blue cells). The concentration of bacterially produced molecules is greatest in distal tumor regions and will remain constant as long as expression of these proteins continues.
Systemically injected (red syringe), passive chemotherapeutic molecules (dark red cubes) diffuse into tumor tissue from blood vessels. The concentration of chemotherapeutic molecules is greatest in systemic blood (before it even gets to the tumor) and drops as it is cleared by the liver or kidneys. Therefore, bacterially produced molecules will be more cytotoxic in the distal and specific tumor regions, and less systemically toxic, compared to passive molecules.
Payload Delivery
The therapeutic payload (drug/cytotoxic agent) can be delivered in the form of DNA, RNA or protein depending on its intended use and the type of delivery bacteria. In the majority of cases, bacterial vectors are transformed to direct the expression of therapeutic proteins in the bacteria. The vectors can be engineered such that the release of the therapeutic payload is once a tumor has been found.
Now, we are focusing on genetically engineering bacteria to perform as transporters for payloads. The selective targeting and crazy growth of bacteria in tumors, minimizes the damage done to healthy cells, which is common for other cancer therapies. Several groups have already began engineering bacteria to produce and transport anticancer toxins, cytokines, and apoptosis-inducing factors.
Therapy with live tumor-targeting bacteria gives us an option to meet the challenges that current therapies can’t. Like I mentioned in the beginning of the article, there are over 100 different types of cancer, and every single tumor behaves differnetly. In comparison to most other therapies, tumor-targetted bacteria is not actually directly affected by the DNA of the tumor, since it begins to attack the tumor from deep inside.
All of this being said, we are still not at the level where we can completely replicate the complicated structure of a bacterial cell. The processes in this article require bacterias to make complex chemicals, perform complex functions, and also know exactly when to turn specific genes on and off.
In effort to do this, Cello is a framework that was built to design computational circuits in living cells. The programming language works by operating a series of gates that create a specific output, when given specific input. Cello converts electronic logic designs to complete DNA sequences encoding logic circuits that can be executed in bacterial cells.
DNA is binary in a couple ways, a given section of DNA is always either:
- Present or absent
- In forward or reverse orientation 🔽
We can assign a 1 or 0 to presence/absence and forward orientation/reverse orientation by making those conditions control expression of a reporter gene (genes that enable the detection or measurement of gene expression).
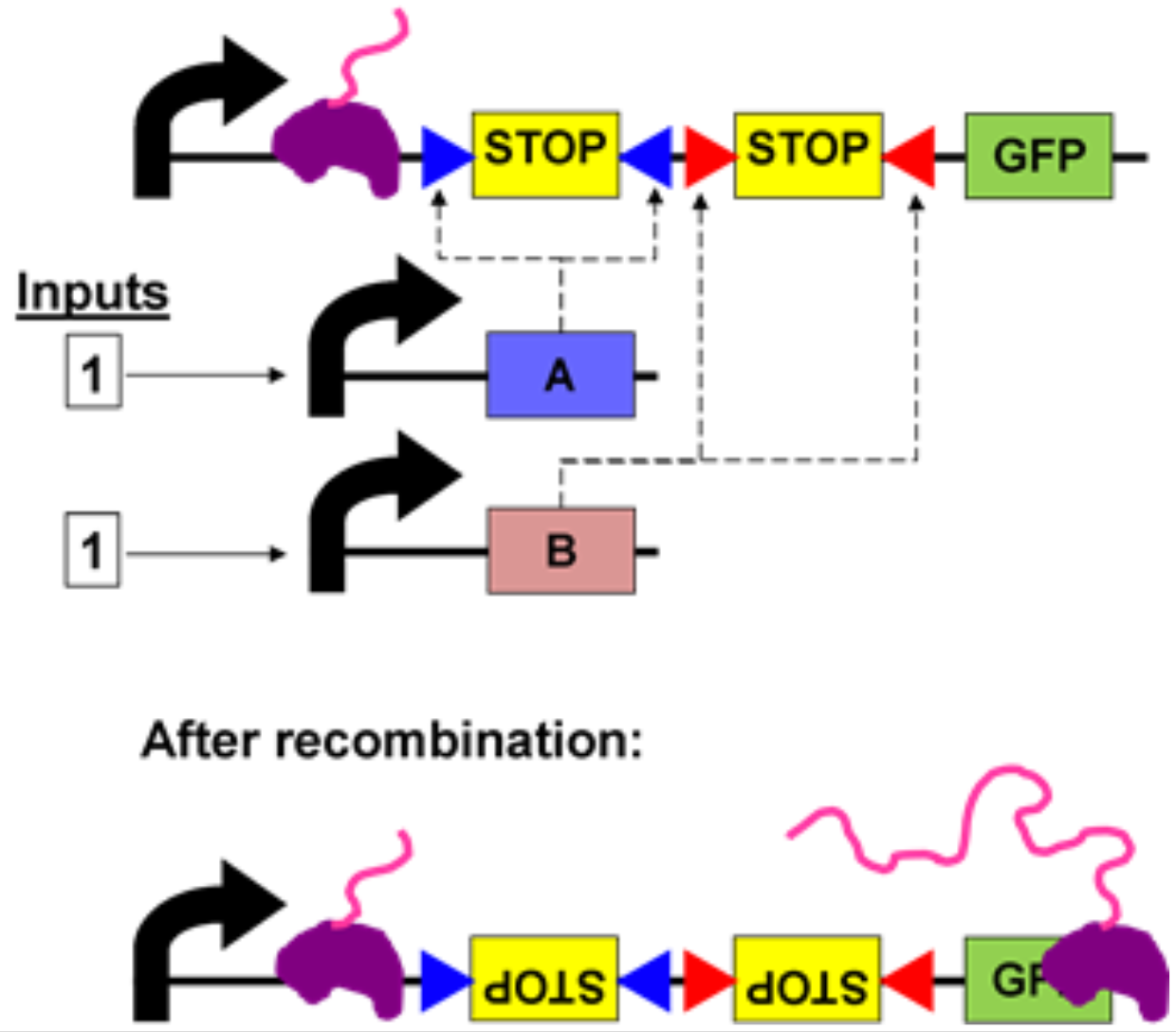
(In reference to the image below). Recombinases (enzymes used to control gene expression) will recognize short, directional sequences of DNA, commonly called “sites,” and usually represented by small triangles (like in the image below). If the sites point the same way, the recombinase cuts and pastes them into a single site, deleting the rest of the DNA in the process. However, if the sites point in opposite directions, the intervening DNA and a portion of the site DNA is flipped, which reverses its original orientation.
Salmonella actually already use this trick to control the expression of genes by flipping the promoter that turns the genes on and off. Hence, the system is built off of that, used by bacteria.
Here’s a quick walkthrough. The above diagram is using only the AND gate, rather than both AND and OR gates. Similar to conditional statements in computer programming, the AND gate produces an output of 1 if both inputs are also (1 AND 1 → produce 1). The genetic AND gate uses two small molecules as inputs, which in turn cause expression of the recombinases that directly activate the logic gate. The output is expression of GFP, causing the E. coli cells to glow green.
At the top of the diagram is the AND gate in its default state: both inputs are 0, as there is no input yet. RNA polymerase (purple blob) is unable to move past the two transcriptional terminators (“STOP”) that together compose the AND gate. When both recombinases are expressed (input: 1,1), the two forward-pointing terminators are flipped independently into reverse orientation. In this new orientation the terminators are unable to block RNA polymerase, and transcription proceeds all the way through the GFP gene, and is then translated into the GFP protein.
This software is the most prevalent to allow us to genetically engineering bacteria to act a certain way when faced with certain situation. Over that last decade, Salmonella, E-Cole and other bacteria have been shown to control tumor growth and promote survival in mice.
Yet, there are still many challenges that come with these new experiments, some of which include:
- Drug production needs to be exactly at the point of high enouch concentration, but not high enough to causing systemic toxicity.
- Live bacteria cannot be sterilized either by heating or by filtering, which presents a major challenge for manufacturing. This also means that the sterility testing on bacteria is not possible. There are several ways we can control this, but it will be very tedius, including purifying rooms and labs to ensure maximum sterility.
- Bacterial cancer therapy is deliberately creating a tumor-destructing infection inside of the body, which may not result in the best way if not taken care of properly.
Initially, synthetic biology critics said that: biology is too complex to rationally design. With Cello, and new discoveries of bacterial therapy, I definitely just proved that wrong.
TL;DR
- Cancer research + treatment discovery has come a long way, but still has a very long way to go
- Cancer is the uncontrollable replication of cells, that basically kill off everything that tells them to stop growing
- Bacteria is so so much more effective that current cancer treatments, since it can specifically target tumors, actively penetrate tissue, are easily detected and can controllably induce cytotoxicity.
- The most recently developed software for bacteria engineering is Cello, which uses a system that is based off of Salmonella.